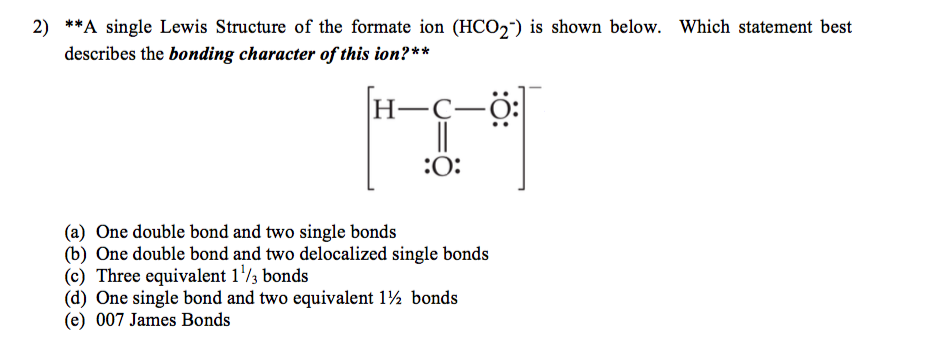
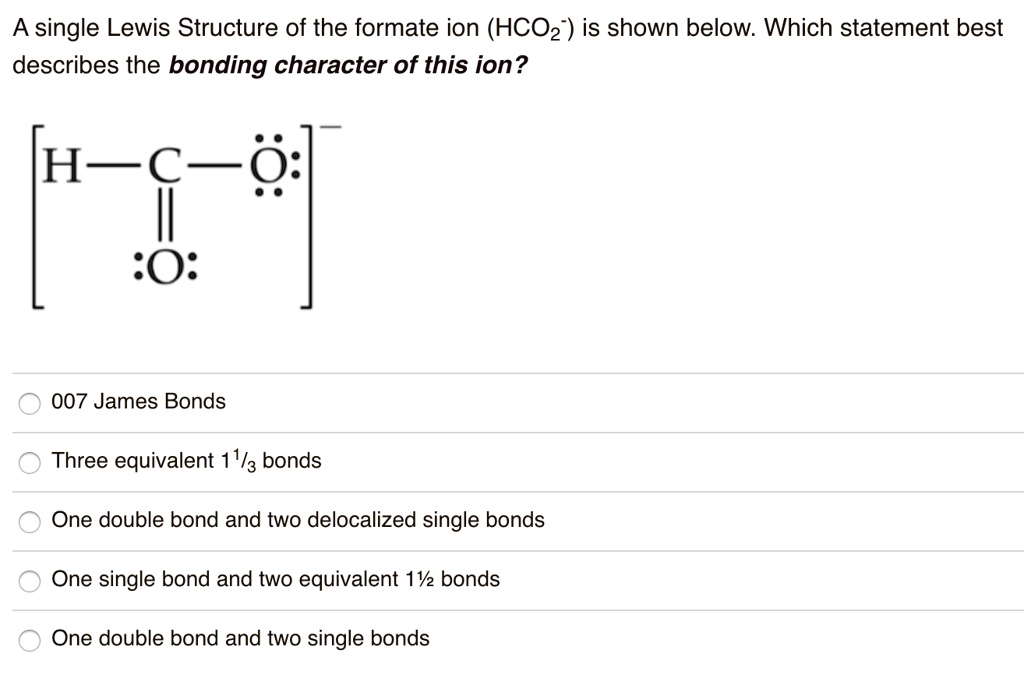
Best Describes the Bonding Character of This Ion
LDFs dipole-dipole hydrogen bonds. So there is a partial polarity created.
When two different atoms with different electronegative values form a covalent bond the atom with a higher electronegative value tends to draw the bonding electrons towards itself.

. A single Lewis Structure of the formate ion HCO-_2 is shown below. Based on the indicated electronegativities arrange the following in order of increasing ionic character. Compare the Lewis structure and bonding of this nitrogen with the nitrogen in the ammonium ion NH 4.
Drawing the Lewis Structure for HCO 2-. A greater than the H-N-H bond angle in ammonia. The extent to which two or more elements share electrons in a bond is known as ionic character.
D The ion has a trigonal pyramidal shape. B The H-O-H bond angle is 120. D there is a very small difference in electronegativity between lithium and iodine.
Hence the geometry of the complex is octahedral and the complex is diamagnetic as there. Which type of bond is formed by the transfer of electrons from one atom to another. A The ion has a tetrahedral shape.
Van der Waals bond. HCO 2-is also called Formate Ion. Which of these statements best describes an integral membrane protein.
B 4 bond pairs and 1 lone pairs. The strength of the ionic bond is directly dependent upon the quantity of the charges and inversely dependent on the distance between the charged particles. Bonding Test Review Astrong covalent bond Bweak ionic bond Cstrong intermolecular force Dweak intermolecular force 30Hydrogen bonding is a type of Aionic Bmetallic Cmolecule-ion Dvan der Waals forces 31Which type of attraction results from the formation of weak momentary dipoles.
Ionic character of a covalent bond is when the electro negativity of the two atoms bonding differs. C there is a very large difference in electronegativity between lithium and iodine. Which compound contains a bond with the least ionic character.
Very hard very high melting point bad conductor. The role of the Zn2 ion is to polarize water into a nucleophile. D If the acetamide hybrid is best represented by contributing structure a predict the H-N-H bond angle.
There are a total of 18 valence electrons in the HCO 2-Lewis structure. A covalent bond B. Argon methane sucrose dry ice.
D 2sp 3 hybridized orbitals of Fe 2 are 6 electron pairs are from CN ion occupy the six hybrid d 2sp 3 orbitals. A One double bond and two single bonds b One double bond and two delocalized single bonds c Three equivalent 1 13 bonds d One single bond and two equivalent 1 12 bonds e 007 James Bonds. Atoms connected in a network of covalent bonds.
B identical H-N-H bond angle in ammonia. Also I would note that the 3 sigma MO has less bonding character and the 4 sigma MO has less anti-bonding character. 8 The bonding in lithium iodide has some covalent character because A the lithium ion polarizes the iodide ion.
A 4 bond pairs. D less than the H-N-H bond angles in ammonia. With HCO 2-youll need to form a double bond between one of the Oxygen atoms and the Carbon atom to fill the octets.
Ligand field theory The disparity between free-ion and complex-ion electronic state energies is the so-called nephelauxeticeffect cloud-expanding which depends upon both the metal ion and ligand. The H-N-H bond angles in the ammonium ion NH4 are. Energy levels Russell-Saunders term states in the complex than predicted for the ion in the crystal field environment.
Ionic Character Trend. Carbon C is the least electronegative atom and goes at the center of the HCO 2-Lewis structure. Using a periodic table seen in the previous section elements which tend to.
Which statement best describes the bonding in A3B. AHF BCH3Cl CCH3F DHCl. The structure that best describes the double bond character of the amide bond in acetamide is shown by the Roman numeral _____ asked Jun 15 2017.
A cation with a 2 charge will make a stronger ionic bond than a cation with a 1 charge. An ionic bond is the electrostatic force that holds ions together in an ionic compound. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities and is the primary interaction occurring in ionic compoundsIt is one of the main types of bonding along with covalent bonding and metallic bondingIons are atoms or groups of atoms with an.
E If on the other hand the acetamide hybrid is best represented by contributing structure b predict the H-N-H bond angle. Up to 24 cash back E a metallic bond 22The bond in an N2 molecule is A the bond between two oppositely charged ions B the bond between small stable atoms like helium atoms C the bond formed when an alkali metal reacts with a halogen D the bond between two nuclei in a sea of electrons E a pair of electrons shared between atoms 23A covalent bond is best. The peptide bond bond has partial _____ character.
Since there are six ligands around the central metal ion the most feasible hybridization is d 2sp 3. An ionic bond 12. C The compound is largely ionic with A as the cation.
The role of the Zn2 ion is to act as a base and abstract a proton from water. A hydrogen bond D. B the iodide ion polarizes the lithium ion.
Which statement best describes the bonding character of this ion. A coordinate covalent bond C. CsBr LaBr3 PBr3 MgBr2.
Note that the total bond order is approximately one because the 3 sigma MO is largely localized on the F atom the 3 sigma MO is not totally bonding and the 1pi MOs are completely localized on the F atom. C The H-O-H bond angle is 90. Which element can react with uorine to form more than one binary compound.
The shape of XeF4 molecules is base on them having. As CN is a strong ligand it causes the pairing of the unpaired 3d electrons. Atoms or Molecules.
Fairly soft low melting point bad conductor.

Covalent Bond Definition Properties Examples Facts Britannica

The Intriguing Case Of The One Photon And Two Photon Absorption Of A Prototypical Symmetric Squaraine Comparison Of Tddft And Wave Function Methods Avila Ferrer 2019 Chemphotochem Wiley Online Library
Ionic Bond Partially Covalent In Nature Chemistry Ionic Bonds

Covalent Compounds Covalent Bond Properties Examples With Videos

Network Rightsizing Best Practices Guide

Chemical Bonding From The Statistics Of The Electron Distribution Pendas 2019 Chemphyschem Wiley Online Library
Solved A Single Lewis Structure Of The Formate Ion Hco 2 Chegg Com

What Is The Difference Between Organic And Inorganic Chemistry Organic Chemistry Chemistry Education Medical Technology

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Molecular Geometry Chemistry
Revisiting The Covalent Nature Of Halogen Bonding A Polarized Three Center Four Electron Bond Rsc Advances Rsc Publishing Doi 10 1039 D1ra05695f

Pushing The Limits Of Chemical Bonding

Ionic Bond Definition Properties Examples Facts Britannica
Molecules Free Full Text Confirming The Molecular Basis Of The Solvent Extraction Of Cadmium Ii Using 2 Pyridyl Oximes Through A Synthetic Inorganic Chemistry Approach And A Proposal For More Efficient Extractants

Inspirational Positive Quotes Friends That Become Family Friends Become Family Quotes Friends Like Family Quotes Friends Are Family Quotes

Molecule Definition Examples Structures Facts Britannica
Solved A Single Lewis Structure Of The Formate Ion Hcoz Is Shown Below Which Statement Best Describes The Bonding Character Of This Ion H C G O 007 James Bonds Three Equivalent I3 Bonds One

Neither Too Classic Nor Too Exotic One Electron Na B Bond In Nabh3 Cluster Pino Rios Angewandte Chemie Wiley Online Library



Comments
Post a Comment